Sepsis currently costs the US healthcare system more than $60 billion annually and consumes almost 50% of available hospital ICU resources. According to the US CDC, sepsis is difficult to diagnose in its early stages because an accurate and reliable IVD test for sepsis does not exist. There are no current lab tests for sepsis that detect the onset of the pathology since they are all based upon biomarkers or events occurring during later stages of sepsis, and have little or no utility in guiding therapy during the initial stage.
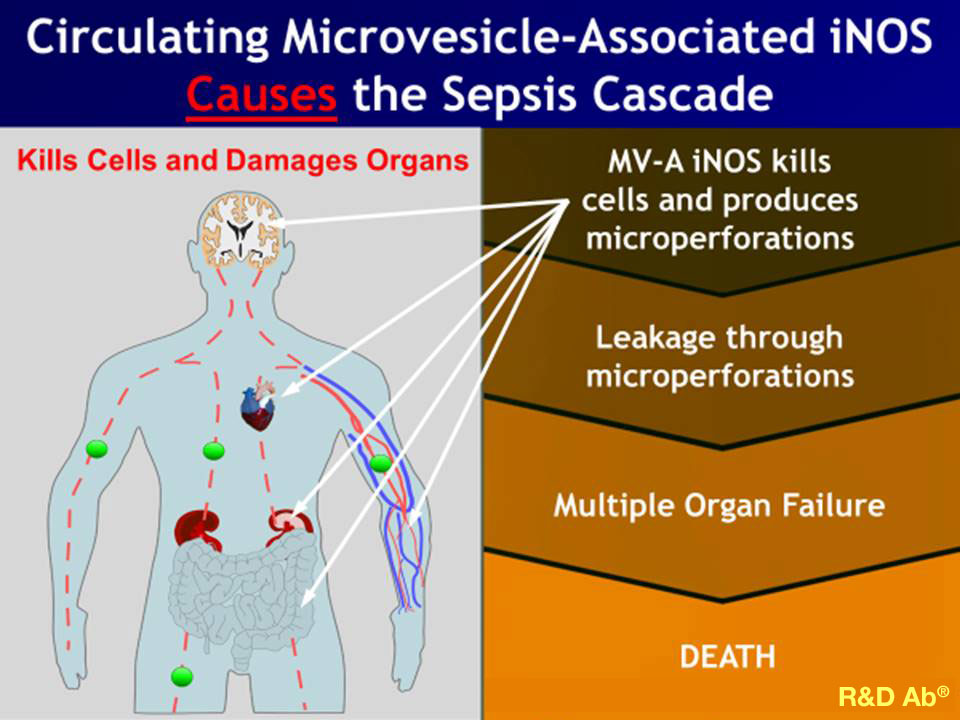
Our clinical data demonstrate plasma iNOS as an excellent biomarker for the onset of sepsis, and our PliNOSa IVD test has high diagnostic sensitivity and specificity for the onset of sepsis pathology. Additionally, clinical data strongly supports plasma iNOS is the causative agent for the organ damage and dysfunction and for vascular leak all of which are the classic hallmarks of the sepsis pathology. Consequently, circulating plasma iNOS is a validated new therapeutic target to treat sepsis. Thus, plasma iNOS is the specific target for our candidate humanized monoclonal antibody aSeptiMab immunotherapeutic to treat the sepsis pathology very early, i.e. before organ damage and dysfunction occur. This is especially relevant during the ongoing COVID-19 pandemic because almost all COVID deaths are due to multiple organ failure caused by sepsis. .